Next-gen digital health innovation in clinical trials
In a world of on-demand everything the biopharma R&D cycle is an anomaly: drugs require an average of 10 to 15 years to progress from discovery to FDA approval, and an average investment of $2.6 billion across the development lifecycle. Never has that lengthy process been felt so acutely than when the world waited on edge while academic institutions, government agencies, and pharmaceutical companies raced to develop a COVID-19 vaccine in record time.
Much has been written about the impact of COVID-19 on clinical trials and the need to shift toward decentralized or virtual clinical trials. With this post, we’ll reflect on the evolution of digital in clinical trials, discuss the current state, and explore the opportunity to further transform drug discovery.
Evolution of Digital in Clinical Trials
Initial efforts to integrate technology into clinical trials focused on electronic data capture systems (EDC), electronic clinical outcome assessments (eCOA), and electronic patient reported outcomes (ePRO). EDC allows for clinical research documents to be stored digitally while eCOA and ePRO enable the digital distribution of assessments and surveys–each serving as a digital tool within a traditional trial. The idea of decentralized or virtual clinical trials—clinical trials without traditional study sites—only emerged in the last decade with a few initial failed attempts—attributed to poor enrollment and engagement—followed by successes from Science37 in partnership with AOBiome in 2017 and Janssen in partnership with PRA Health Sciences in 2019.
More recently, the infusion of digital health solutions into traditional clinical trials and the pace of decentralization have accelerated. Much of this innovation has come from startups, which have steadily been raising capital in recent years—consistently securing more than $300M annually since 2016. So far through Q3 2020 , digital health solutions targeting clinical trials have raised $787M, skyrocketing well above the prior high of $403M in 20161. This increase in funding is attributed to a few large rounds—also resulting in a higher average deal size—rather than an increase in the total number of deals compared to previous years. We presume the spotlight on decentralized clinical trials in light of COVID-19 was a driving force for many investors. Through Q3 2020, the largest funding rounds for clinical trial solutions have been ConcertAI with $150M, Verana Health with $100M, Aetion with $82M, and THREAD with $50M.
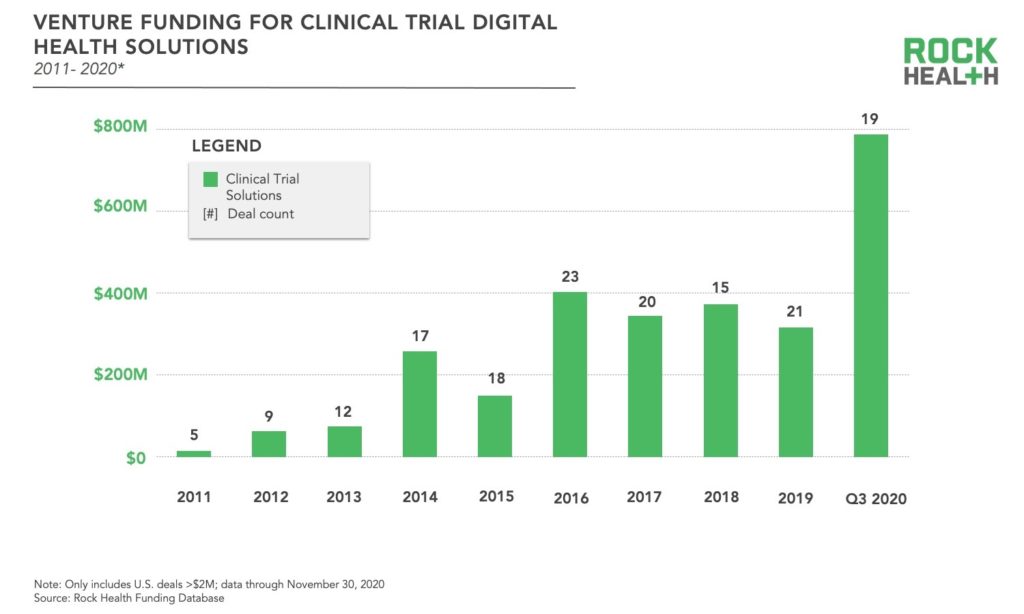
The FDA has also spurred increased adoption of digital health solutions for clinical trials via policy changes. First, the FDA passed the 21st Century Cures Act supporting data interoperability and real world evidence with implications for regulatory approvals, then in 2018 former FDA Commissioner Scott Gottlieb called for rethinking clinical trials. Most recently the FDA launched a digital health center of excellence furthering the agency’s overarching dedication to the advancement of digital health technology, including those technologies used in research.
The Digital Clinical Trial Landscape
All stages of the clinical trial lifecycle—study set-up, activation, and evaluation—are being touched by digital health innovation. Solutions can be segmented along those stages, and while the majority of startups are focused on delivering solutions in one stage, a few are offering end to end solutions.
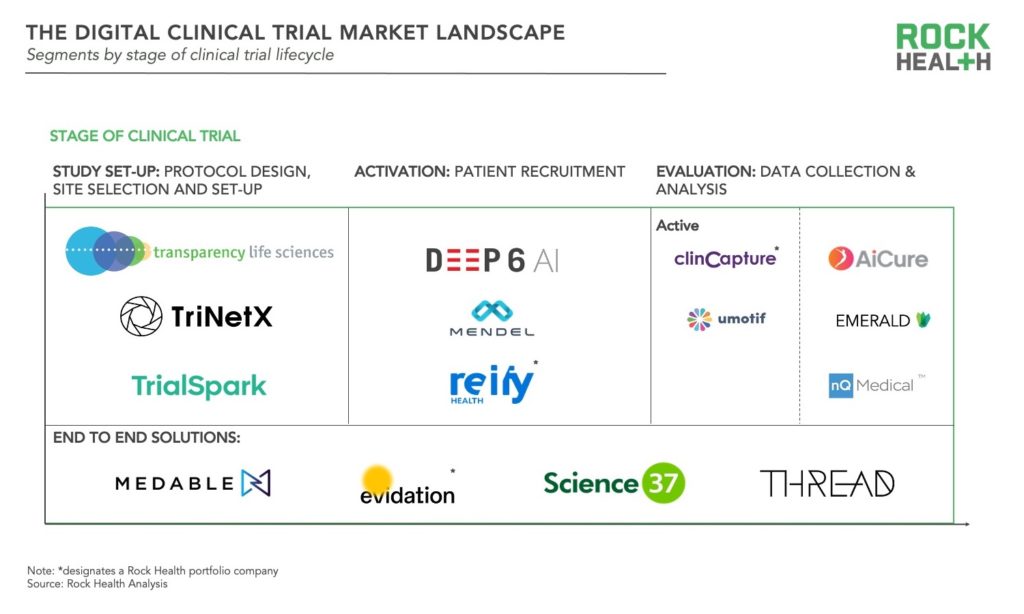
- Study set-up: These solutions target the planning stage of clinical trials, from protocol development and approval to site-selection and set-up. Although pharma, medtech, and CROs are well versed in protocol design, desire for trials to be more patient centered has resulted in solutions to crowdsource opinions of patients, caregivers, and health professionals such as that of Transparency Life Sciences. Other tools such as TriNetX inform site selection based on localization of patients who match protocol criteria. Another company, TrialSpark uses big data analytics to identify high-potential locations where trial participants can be found. Lastly some companies are focused on increasing the efficiency of training for investigators and staff prior to site launch.
- Activation: These digital health products enable sponsors to more quickly identify, screen, and enroll participants for clinical trials. More than two-thirds of trial sites fail to meet initial recruitment targets and recruitment accounts for roughly 40% of the collective clinical trial budget of U.S. pharma companies. Many solutions rely on algorithms, artificial intelligence (AI), and/or natural language processing (NLP), but approach varies. Some solutions use NLP to match patients to trials based on their medical records such as Deep6AI or Mendel.AI. Other companies have developed platforms to provide real time insights and drive recruitment efficiencies for trial sites and sponsors as in the case of Reify. More recently, efforts have emerged to improve diversity and inclusion in clinical trial recruitment, for instance through the collection and de-identification of patient data with a focus on minority communities from DrugViu.
- Evaluation: These solutions collect and manage patient data associated with outcomes—either actively or passively. Active data collection encompasses the categories of digital health companies which initially emerged for clinical trials: electronic data capture (EDC), electronic clinical outcome assessments (eCOA), and electronic patient reported outcomes (ePROs). Companies with data capture platforms include Clincapture and uMotif. More recently, passive data collection has become more prominent, leveraging sensors in mobile phones and various biosensors. Some solutions utilize smartphone cameras to capture data such as ingestion of medication (as in the case of AiCure). Another set of companies track movement in the home such as Emerald, while others monitor phone usage to track conditions. For example, nQmedical uses typing and touch screen kinematics to assess neurodegeneration.
- End-to-end: These solutions cover the full spectrum of stages and enable facilitation of decentralized or virtual trials. Evidation, Medable, Science 37, and THREAD are some of the key players offering end-to-end solutions. Evidation leverages their patient community Achievement and digital platform to execute decentralized or virtual trials as in the recently published study of Omada Health’s chronic disease management program. All of these companies are actively partnering with pharma and/or contract research organizations (CROs), underscoring the interest of enterprise healthcare organizations in shifting to decentralized trials. For example, Medable has partnered with LabCorp, Science37 has partnered with Boehringer Ingelheim and Novartis, and THREAD has also partnered with Novartis.
The Next Horizon of Digital Clinical Trials
Digital transformation of clinical trials is already underway. Rather than doing the same things more efficiently vanguard companies are looking for ways to use technology to do things differently. Some of these solutions are right around the corner, while others are further off. Below are some of the most exciting developments we are tracking in the space:
- Optimize the success of clinical trials before they even start
One opportunity for meaningful innovation exists even before a clinical trial starts by optimizing the likelihood of success. One such approach, offered by Intelligencia.AI, leverages artificial intelligence to predict the likelihood of success and adapt design features to de-risk the trial. Another approach undertaken by GNS Healthcare uses AI to create “in silico” patients and pinpoint which characteristics of a patient are best suited for a given clinical trial. - Replace traditional control arms with synthetic control arms or digital twins
Traditionally, control groups in randomized controlled trials will receive a placebo or the current therapy considered standard-of-care to serve as a comparison for the intervention under investigation. However, the use of traditional control groups has raised ethical questions especially for debilitating conditions such as cancer or rare disease, or when the standard of care has limited efficacy. One alternative is synthetic control arms which use historic data or real-world evidence as opposed to traditional control arms. One digital health startup, UnlearnAI, is taking the idea a step further with their machine learning platform DiGenesis that builds “digital twins.” In this context, digital twins are comprehensive, longitudinal, and computationally generated clinical records that describe what would have happened if a patient had received a placebo. - Real-world data and real-world evidence as a way to achieve regulatory approval
Real-world data (RWD) and real-world evidence (RWE) is generated during routine clinical care outside of the confines of randomized controlled trials. The 21st Century Cures Act placed focus on the use of RWD and RWE in relation to regulatory decisions, opening the door for its use to support follow-on indications for approved drugs. Flatiron Health, acquired by Roche in 2018 for $1.9B, offers a RWE platform for oncology and has partnered with both the FDA and NICE to further the use of RWE in regulatory decisions for oncology. Other notable companies using RWD to support later stage R&D include ConcertAI, also focused in oncology, and Aetion, which uses AI to generate RWE from medical and pharmacy claims data and has partnered with the FDA to re-create randomized clinical trials (RCTs) through RWE. We expect COVID-19 to further accelerate the use of RWE in regulatory decisions as interventions made available via emergency use authorization have been afforded the opportunity to collect RWD in an unprecedented way. - Breathe new life into pipeline assets
Despite the significant time and financial investment, approximately 50% of phase 3 clinical trials are doomed to fail, and the percentage only increases when considering earlier phases. However, advanced analytics and algorithms are now being leveraged to rescue clinical trials on the brink of failure—even those that have already failed. The most common approach—advanced by Healx, a UK-based startup focused on rare disease, and Netramark—uses AI to identify alternative indications or specific subpopulations for which an asset may be well suited.
How to Ride the Wave of Innovation
The explosion of innovation in the space—while exciting—can be bewildering. As a kid in a candy store (or a biopharma or digital health company thinking about your evidence generation plan), where do you start? First, figure out where you are in the journey of incorporating digital solutions into clinical trials. Next, develop a strategy to ensure your evidence generation is on par with the first wave of digital health innovation. Then, identify which therapeutic areas or specific pipeline assets could most benefit from reimagining the traditional approach and begin your transformation process.
We’re excited for the next horizon of digital health innovation to bring treatments to patients more quickly and effectively.
Footnotes
1Rock Health Venture Funding Database


