Imagining a world free of diabetic foot ulcers: Our investment in Podimetrics
We’ve been supporting passionate entrepreneurs seeking to make healthcare massively better since our inception over eight years ago. In Rock Health’s early days, we helped build and shape dozens of companies as a leading digital health accelerator. In 2013 we evolved our model to better support founders by investing directly in high-impact solutions improving the quality, safety, and accessibility of care to our healthcare system. We continue to do so today—and our relationship with our newest investment is unique in that it follows this same evolution from accelerator to investor. We’re thrilled to announce our participation in Podimetrics’ $13.4M Series B.
Podimetrics first joined the Rock Health family in May 2012 as a member of the accelerator program’s Boston cohort—and our first class of companies outside of San Francisco. The founders of Podimetrics were just beginning their journey to help prevent diabetic foot ulcers (DFUs), which are open sores or wounds that typically develop on the bottom of the foot as diabetes progresses. Over the course of the program, they collaborated with our advisors and physician mentors to discuss product development, FDA regulatory strategies, and clinical protocols to ultimately bring their vision to life. Their time in our accelerator program culminated with the signing of their initial term sheet from a local Boston-area venture capital fund, Norwich Ventures. Today we’re proud to invest alongside Norwich Ventures and Scientific Health Development in Podimetrics’ latest round.

The Podimetrics team during Rock Health’s Boston accelerator program (before we became a venture fund!) in 2012.
Since 2012, Podimetrics has worked tirelessly on a remote patient monitoring (RPM) solution to tackle the devastating problem of diabetic foot ulcers. The International Diabetes Federation reports one in every three dollars spent on diabetes care is linked to DFUs. The incidence rate of DFUs is greater than 6% globally among diabetic populations—and in the US alone, between 1M and 3.5M people have a history of foot ulcerations. The effects on quality of life are substantial, due in part by how quickly the condition can escalate. DFUs often lead to debilitating amputations and a 2.5X increased mortality risk five years after the patient’s first episode. However, the development of foot ulcers is largely preventable.
Remotely managing chronic conditions is no simple task—and as we noted in our Q1 funding update, we’ve seen a number of recent business models in the RPM space that focus on capturing revenue from newly unbundled reimbursement codes without first validating the patient outcomes from the solution itself. But we’re interested in funding RPM technologies that have generated clinically validated outcomes data and cost savings to their customers by focusing on prevention of acute and chronic conditions.

Podimetrics’ SmartMat™ collects foot temperature scans, which are then sent to Podimetrics and the care team for analysis and review.
In particular, Podimetrics combines a number of factors to ensure the success of its RPM solution:
- Clinical-grade sensor technology that integrates into the patient lifestyle via its FDA-cleared SmartMat, which patients simply stand on for 20 seconds to obtain an accurate reading of foot temperature to identify “hotspots” where ulcer inflammation is at highest risk
- Predictive analytics run on a HIPAA-compliant, cloud-based server that ingests and analyzes foot temperature readings through its proprietary algorithms
- Best-practice clinical protocols and care coordination by alerting patients and their providers of concerning results so they can work together to avoid DFUs by implementing preventative interventions at a lower cost
By bringing together these components, we believe Podimetrics can deliver on the aim of bringing predictive, proactive care coordination to patients—and provide this care at higher quality and lower costs.
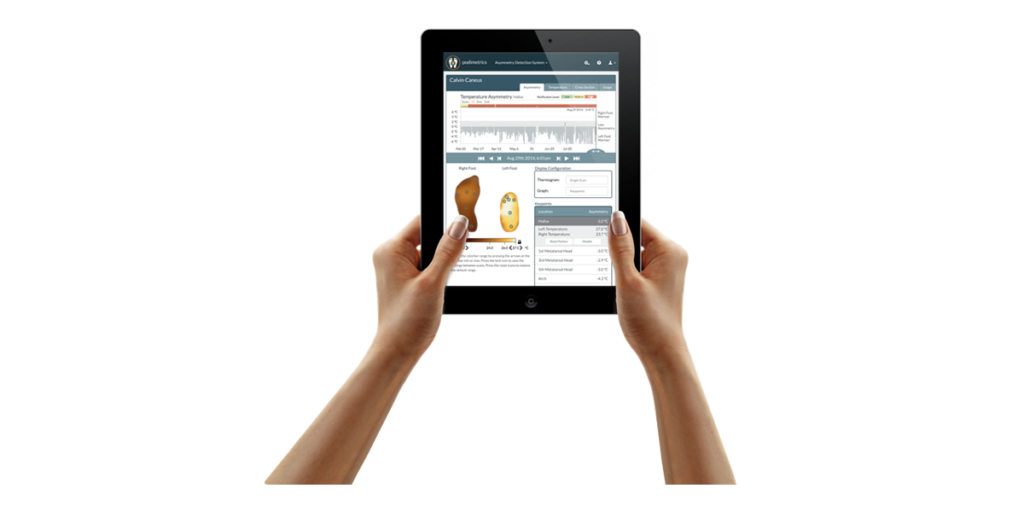
Podimetrics offers online tools for clinical decision support and population management so providers can triage and focus on the patients who need care most.
The Podimetrics team has both validated their SmartMat hardware with the FDA and proven their clinical interventions and care coordination through numerous studies and commercial programs with payers and providers. In 2017, Podimetrics released a 129-patient study published in Diabetes Care demonstrating the SmartMat achieved 97% sensitivity in predicting the emergence of DFUs an average of five weeks prior to clinical presentation of the ulcer. Moreover, the study found 86% of patients used the SmartMat at least three times per week and 88% reported it was easy to use.
With clinically validated sensor technology and care coordination, Podimetrics is now launching its product to additional commercial payers and at-risk providers. In December 2018, Podimetrics was included in the Veterans Affairs (VA) System’s national guidance documents for high-risk patients and is now in use at 14 VA hospitals in the US.
We could not be more proud of the work the Podimetrics team has accomplished over the last seven years, and we’re excited to continue working alongside CEO Jon Bloom and his team to help patients lead healthier lives free of DFUs!
